Have you ever stopped to wonder what everything in the universe, from the air you breathe to the phone you’re holding, is actually made of? The answer lies in the fascinating world of chemistry, where scientists have developed a system for classifying matter. It might sound complex, but fear not, because this guide will break down the classification of matter in a way that’s clear, engaging, and, dare we say, even fun.
Imagine yourself as a detective tasked with organizing a giant warehouse filled with… well, everything! From shimmering diamonds to fluffy clouds, you need a system to categorize these vastly different materials. That, in essence, is what the classification of matter does. It provides a framework for understanding the composition and behavior of all matter in the universe.
So, grab your magnifying glass (figuratively speaking), and let’s delve into the thrilling world of classifying matter!
The Big Two: Pure Substances vs. Mixtures
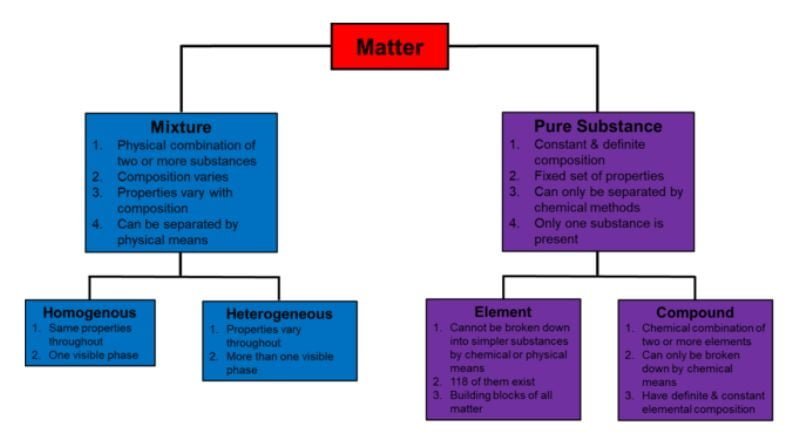
The first major division in the classification of matter separates it into two broad categories: pure substances and mixtures.
- Pure Substances: These are the stars of the show, like the diamonds in our warehouse analogy. Pure substances have a constant and uniform composition throughout. This means every single part of a pure substance is exactly the same in terms of its chemical makeup and properties. Think of a bag of pure table salt (sodium chloride). Every tiny grain of salt in that bag is identical, with the same ratio of sodium and chlorine atoms and the same characteristic salty taste.
There are two main types of pure substances:
- Elements: These are the fundamental building blocks of matter, like the basic ingredients in a recipe. Elements cannot be broken down further by chemical means into simpler substances. Examples include gold (Au), oxygen (O2), and hydrogen (H2).
- Compounds: These are formed when two or more elements chemically combine in a fixed ratio. Unlike elements, compounds can be broken down into their constituent elements through chemical reactions. Water (H2O), carbon dioxide (CO2), and sugar (C6H12O6) are all examples of compounds.
- Mixtures: Now, imagine that warehouse filled with various items jumbled together – that’s the world of mixtures. Mixtures are combinations of two or more different substances that are not chemically combined. The key here is that the components of a mixture retain their individual chemical identities. For instance, a bowl of trail mix is a mixture of nuts, raisins, and chocolate chips. Each element remains distinct, and you can easily pick out a single raisin without affecting the other ingredients.
There are two main types of mixtures:
- Homogeneous Mixtures: These are the well-organized sections of our warehouse, where items are evenly distributed throughout. In a homogeneous mixture, the components are so uniformly mixed that they appear like a single substance. Think of a cup of tea – the tea leaves and water are evenly distributed, giving the tea a consistent color and taste throughout.
- Heterogeneous Mixtures: These are the messy parts of the warehouse, where things are not neatly arranged. Heterogeneous mixtures have visibly distinguishable components. A good example is a pizza – the crust, cheese, and toppings are easily identifiable, and the mixture is not uniform throughout.
Separating the Unseparable: Physical vs. Chemical Changes
Now that we’ve categorized matter by its composition, let’s explore how these categories can be further distinguished based on how their components interact. Here’s where the concepts of physical and chemical changes come into play.
- Physical Changes: Imagine rearranging the items in our warehouse without altering them fundamentally. Physical changes involve a change in the physical appearance or state of matter, but the chemical composition remains unchanged. For example, crushing an ice cube into smaller pieces is a physical change. The ice is still H2O, just in a different form. Similarly, boiling water changes its state from liquid to gas, but the water molecules themselves are still intact.
- Chemical Changes: Now, picture taking those warehouse items and transforming them into something entirely new. Chemical changes involve a rearrangement of atoms, resulting in the formation of new substances with entirely different properties. When wood burns, it undergoes a chemical change. The wood, composed of complex molecules, reacts with oxygen to form new substances like carbon dioxide and water vapor.
Understanding the difference between physical and chemical changes is crucial in classifying matter. Mixtures can often be separated into their components through physical means like filtration or distillation, while separating the components of a compound typically requires a chemical reaction.
Beyond the Basics: Advanced Classification of Matter
The classification of matter doesn’t stop at pure substances and mixtures. Chemists have further subcategories to account for the diverse ways matter can exist. Here are a few interesting examples:
- Colloids: Imagine a particularly tricky section of our warehouse filled with a mixture where the components are neither truly dissolved nor separate. Colloids are a special type of mixture where tiny particles (ranging from 1-1000 nanometers) are dispersed throughout another substance. Milk is a classic example of a colloid – the fat particles are suspended throughout the water without completely dissolving or settling out. Fog and mayonnaise are other examples of colloids.
- Suspensions: These are heterogeneous mixtures where larger particles (greater than 1000 nanometers) are dispersed throughout a liquid. Unlike colloids, these particles are eventually visible to the naked eye and will eventually settle out if left undisturbed. A muddy puddle is a prime example – the clay particles are suspended in the water but will eventually sink to the bottom. Salad dressing with visible pepper flakes is another example.
- Solutions: Ah, the neatly organized shelves of our warehouse! Solutions are homogeneous mixtures where one substance (the solute) is dissolved in another (the solvent). The solute particles are evenly distributed throughout the solvent and are typically too small to be seen with the naked eye. Saltwater is a solution of sodium chloride (solute) dissolved in water (solvent). Air is another example – various gases (solutes) are dissolved in nitrogen (the main solvent).
- Plasmas: Step outside the warehouse and into the vastness of space! Plasma, often referred to as the “fourth state of matter,” is a hot, charged gas composed of free electrons and ions. Unlike the other classifications, plasma isn’t encountered very often in our daily lives. However, it’s the most abundant form of matter in the universe, making up stars and the material between stars.
Putting it All Together: How Classification Helps Us Understand the World
So, why is the classification of matter such a big deal? It’s all about understanding the world around us. By classifying matter, chemists can:
- Predict Properties: Knowing the classification of a substance allows scientists to predict its physical and chemical properties. For example, understanding that sodium chloride is an ionic compound helps explain its solubility in water and its ability to conduct electricity.
- Design Materials: The classification system guides the development of new materials with specific properties. By understanding how different types of matter interact, scientists can design alloys, polymers, and other materials for various applications.
- Separate Components: The classification system informs methods for separating mixtures into their constituent parts. For instance, knowing that oil and water are immiscible liquids (don’t mix) allows us to use techniques like separation funnels to isolate them.
- Control Reactions: Understanding the classification of reactants and products in a chemical reaction helps scientists control the reaction and optimize desired outcomes. For instance, knowing that baking soda (a base) reacts with vinegar (an acid) allows us to predict the formation of carbon dioxide gas and control the amount produced.
In conclusion, the classification of matter is not just a fancy way to organize stuff in a warehouse. It’s a powerful tool that unlocks the secrets of the material world around us. From the air we breathe to the materials we use every day, understanding classification empowers us to predict, design, separate, and control the matter that shapes our universe. So, the next time you look at something, take a moment to appreciate the fascinating world of classification – a system that helps us demystify the very stuff of existence.