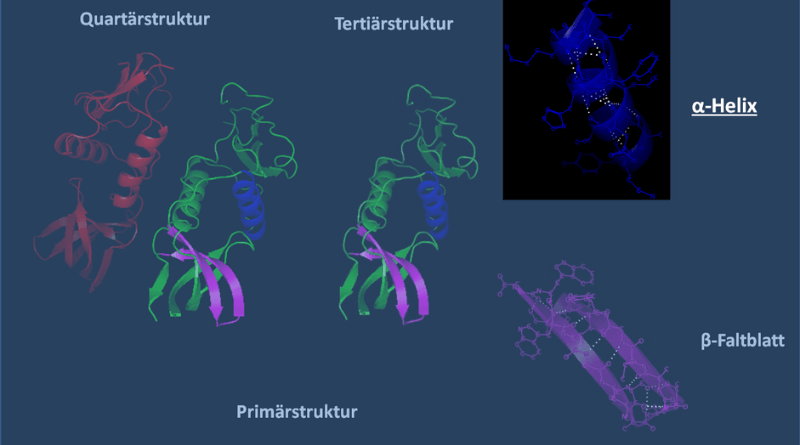
In the intricate realm of protein structures, behold the marvel that is an Alpha Helix—a structural feature of proteins. Picture a coiled spectacle where the polypeptide backbone elegantly forms a right-handed helix. Within this molecular ballet, stability is woven through the delicate dance of hydrogen bonds, gracefully linking the carbonyl oxygen of one amino acid with the amide hydrogen of another amino acid, strategically positioned four residues away along the peptide chain.
Structural Symphony of the Alpha Helix
The alpha helix unfolds as a regular structure, revealing a repetitive unit of 3.6 amino acids. Behold the polypeptide backbone, gracefully curving into a right-handed helix, with a pitch of 5.4 Å and a rise of 1.5 Å per amino acid. The amino acid side chains, akin to celestial adornments, extend outward from the helical embrace.
The ethereal stability of the alpha helix is orchestrated by intramolecular hydrogen bonds. Witness the poetic connection between the carbonyl oxygen of one amino acid and the amide hydrogen of another amino acid, delicately interwoven across the peptide chain—a ballet within the confines of a single polypeptide.
Functional Choreography of the Alpha Helix
Alpha helices, these molecular virtuosos, grace a myriad of proteins, from the structural sentinels to the enzymatic maestros and the transport facilitators. Their roles are as diverse as the proteins they inhabit:
- Sculptors of Protein Edifice: Alpha helices sculpt and maintain the grandeur of protein structures.
- Enzymatic Ballet: The active sites of enzymes often feature the elegance of alpha helices.
- Molecular Transport Pas de Deux: Alpha helices pirouette through the transport of molecules across cell membranes.
Behold the gallery of proteins adorned with alpha helices:
- Myoglobin Masterpiece: Myoglobin, the guardian of oxygen in muscle cells, adorned with eight alpha helices.
- Hemoglobin Symphony: In the bloodstream ballet, hemoglobin, a quartet of subunits, each boasting eight alpha helices.
- Collagen Tapestry: Weave through the connective tissues, where collagen, a structural masterpiece, intertwines three alpha helices in a mesmerizing twist.
Alpha Helix Anomalies
Yet, in the cosmic ballet of proteins, anomalies arise. Mutations, the disruptors of this molecular harmony, can lead to afflictions. Consider the tragic tale of mutations affecting the alpha helix structure within the hemoglobin protein— a narrative that unfolds as sickle cell anemia. This genetic saga transforms red blood cells into sickle-shaped protagonists, diminishing their ability to carry oxygen, a deviation that begets myriad health tribulations.
In the grand tapestry of proteins, the alpha helix emerges as a recurrent structural motif, conducting a symphony of roles from maintaining structural integrity to orchestrating enzymatic prowess and facilitating molecular transport. However, in this molecular ballet, mutations may cast a shadow, giving rise to ailments that disrupt the harmonious choreography of the alpha helix.