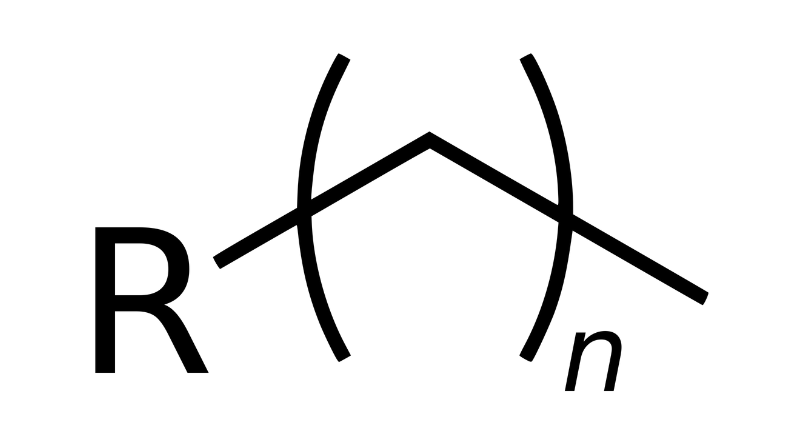
In the intricate realm of organic chemistry, alkyl groups emerge as a fascinating species of organic functional groups. Comprising a saturated carbon chain adorned with hydrogen atoms, they epitomize simplicity yet omnipresence in a myriad of organic compounds—ranging from alkanes and alkenes to alkynes and alcohols.
To delve into the nomenclature of alkyl groups, one undertakes the intriguing task of shedding the -ane suffix from the corresponding alkane, subsequently donning the -yl suffix. For instance, the progeny of methane (CH4) manifests as the alluringly named methyl (CH3), while ethane’s (C2H6) offspring boasts the moniker ethyl (C2H5), and so forth.
Varieties of Alkyl Groups
The taxonomic classification of alkyl groups unfolds across four distinct echelons, contingent upon the number of carbon atoms affiliating with the pivotal carbon atom bonded to the parent molecule:
- Primary Alkyl Groups: Solely tethered to one other carbon atom, examples encompass the demure methyl (CH3), the suave ethyl (C2H5), and the modest propyl (C3H7).
- Secondary Alkyl Groups: Affixed to two carbon compatriots, representatives include the sophisticated isopropyl (CH3CHCH3) and the dignified sec-butyl (CH3CH2CH3).
- Tertiary Alkyl Groups: Entwined with the allegiance of three carbon confidants, this echelon boasts the esteemed tert-butyl (CH3C(CH3)3) and the opulent isopentyl (CH3CH2CHCH3).
- Quaternary Alkyl Groups: Bestowed with the honor of four carbon comrades, exemplars include the regal neopentyl (CH3C(CH3)3) and the resplendent adamantyl (C10H15).
Characteristics of Alkyl Groups
In the realm of polarity, alkyl groups generally sway towards non-polar proclivities, enveloped in a shroud of hydrophobicity. This inclination dictates their disdain for aqueous environments, opting instead for the solace of non-polar solvents. Notably, their reactivity takes a back seat compared to their organic functional counterparts.
The Utilitarian Ballet of Alkyl Groups
The versatility of alkyl groups finds expression in an array of products and applications, weaving their influence into diverse domains:
- Fuels: At the heart of gasoline and various fuels, alkyl groups play a pivotal role in the energetic dance of combustion.
- Solvents: Within the alchemical concoctions of hexane and toluene, alkyl groups lend their prowess to the realm of solvents.
- Plastics: The orchestration of polyethylene and polypropylene involves the choreography of alkyl groups, contributing to the symphony of plastic production.
- Pharmaceuticals: In the medicinal tapestry, alkyl groups make cameo appearances in the likes of ibuprofen and acetaminophen, adding their signature to the therapeutic narrative.
- Fragrances and Flavors: The olfactory and gustatory realms witness the influence of alkyl groups, infusing their essence into myriad fragrances and flavors.
Everyday Alkyl Encounters
In the tapestry of daily existence, alkyl groups reveal themselves in subtle yet impactful ways:
- The methyl group (CH3) graces the banquet of foods and beverages, adorning fruits, vegetables, and the ubiquitous cup of coffee.
- The ethyl group (C2H5) takes center stage in ethanol, the libation of alcoholic beverages, weaving its presence into the social fabric.
- The propyl group (C3H7) assumes a solvent guise in propanol, quietly contributing to the alchemical realms of dissolution.
- The butyl group (C4H9) finds its place in butane, the stalwart companion of lighters and grills, adding its combustible charm to everyday tasks.
- The isopropyl group (CH3CHCH3) dons the mantle of isopropyl alcohol, standing as a sentinel in the realm of disinfection.
- The sec-butyl group (CH3CH2CH3) graces the world of paints and varnishes as sec-butanol, infusing its essence into the hues of creativity.
- The tert-butyl group (CH3C(CH3)3) takes a spirited leap into the realm of gasoline additives, leaving its mark on the energetic ballet of combustion.
- The neopentyl group (CH3C(CH3)3) orchestrates the solvent serenade in neopentane, contributing to the alchemy of plastics and rubber production.
- The adamantyl group (C10H15) takes on a role of significance in adamantane, a compound donned in the battle against influenza and HIV/AIDS.
In the grand tapestry of organic chemistry, alkyl groups emerge as unsung heroes, weaving their intricate dance across fuels, solvents, plastics, pharmaceuticals, fragrances, flavors, and the subtle nuances of everyday life. Their non-polar demeanor, hydrophobic tendencies, and measured reactivity contribute to their enigmatic allure in the alchemical symphony of compounds and applications.